Living with Crohn’s disease means dealing with more than just stomach pain. It’s a lifelong battle with inflammation that can strike anywhere from your mouth to your anus. For many, it starts with cramps, diarrhea, and fatigue-symptoms that come and go, making it hard to plan anything. But the real problem isn’t just the flare-ups. It’s what happens when inflammation doesn’t stop. The gut wall thickens, ulcers deepen, and tunnels called fistulas form. Strictures narrow the intestines. Some people end up in the hospital. Others need surgery. The good news? The way we treat Crohn’s has changed dramatically in the last 25 years. Biologic therapy is now at the center of managing this disease-and for many, it’s the difference between living in pain and living normally.
What’s Really Going On Inside?
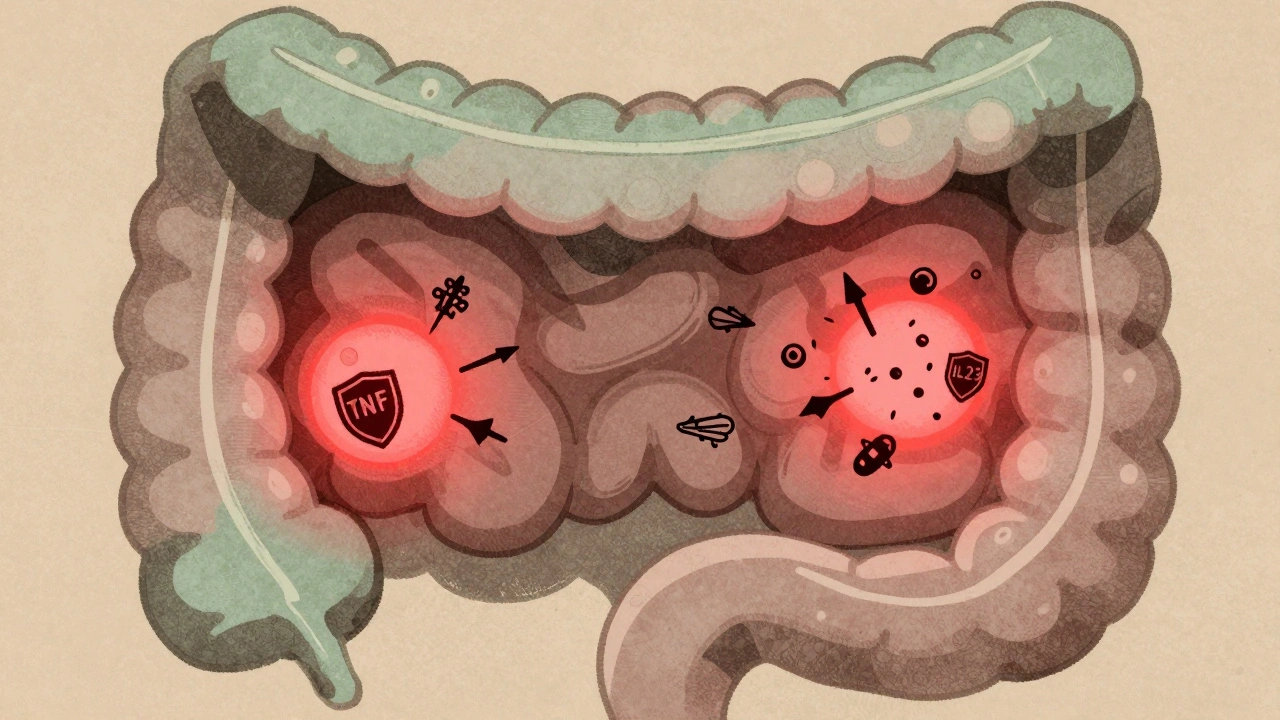
Crohn’s isn’t caused by bad food or stress. It’s an autoimmune condition where the body attacks its own gut. In people with Crohn’s, the immune system mistakes harmless bacteria in the intestines for invaders. This triggers a never-ending inflammatory response. Immune cells flood the intestinal wall, releasing chemicals like TNF-alpha, IL-12, and IL-23. These aren’t just minor players-they’re the main drivers of damage. Over time, this inflammation eats through layers of the gut, leading to scarring, strictures, and fistulas. About 30% to 50% of patients develop strictures within 10 years. Nearly a third end up with fistulas. These aren’t rare complications. They’re common outcomes if inflammation isn’t controlled.How Biologics Turn Off the Inflammation
Biologic therapies are made from living cells, not chemicals. They’re designed to block specific parts of the immune system that cause damage. Unlike older drugs like steroids or azathioprine, which suppress the whole immune system, biologics are like precision tools. They target just the troublemakers. There are two main types used for Crohn’s today. The first group blocks TNF-alpha-proteins that act like alarm bells for inflammation. These include infliximab (Remicade), adalimumab (Humira), and certolizumab (Cimzia). The second group targets different pathways. Vedolizumab (Entyvio) stops immune cells from even getting into the gut. It’s like putting up a barrier at the door. Ustekinumab (Stelara) shuts down IL-12 and IL-23, two other key inflammation signals. Studies show anti-TNF drugs bring remission in 30% to 40% of patients within weeks-twice the rate of placebo. Vedolizumab works slower but has fewer side effects. Ustekinumab hits remission in about 40% of patients by week 8. And here’s the kicker: these drugs don’t just reduce symptoms. They heal the gut lining. Up to 60% of patients on biologics show mucosal healing on follow-up scopes. That’s huge. Healing the lining means fewer flares, fewer surgeries, and better long-term outcomes.Who Gets Biologics-and When?
Not everyone with Crohn’s needs a biologic right away. But the old way of waiting until other treatments fail is outdated. Experts now recommend starting biologics early if you’re at high risk. That means if you have deep ulcers, fistulas, perianal disease, or a family history of surgery. Dr. Jean-Frédéric Colombel’s research shows patients who start biologics early cut their surgery risk by half over five years. Guidelines from the European Crohn’s and Colitis Organisation (ECCO) say anti-TNF drugs are the first choice for moderate to severe disease. But if you have joint pain, skin rashes, or liver issues linked to Crohn’s, vedolizumab might be better. It doesn’t affect the rest of the body like anti-TNFs can. And if you’ve tried an anti-TNF and it stopped working, ustekinumab is often the next step.How They’re Given and What It Costs
Biologics come in two forms: infusions and injections. Infliximab and vedolizumab are given through an IV at a clinic, usually every 8 weeks. Adalimumab and ustekinumab are self-injected under the skin every 1 to 2 weeks. Most patients learn to do it themselves after a few practice sessions with a nurse. But the cost is real. Annual prices range from $35,000 to $70,000 depending on the drug. Adalimumab runs about $40,000-$55,000. Ustekinumab can hit $70,000. Insurance often covers it, but prior authorizations can take weeks. Some patients delay doses because their copay is over $150 per injection. A 2023 survey found 40% of people skipped or postponed treatment because of cost. Biosimilars are starting to change that. Inflectra and Renflexis are cheaper versions of infliximab. They work the same way but cost 15% to 30% less. More are coming. That could make biologics accessible to many more people in the next few years.Side Effects and Risks
Biologics aren’t risk-free. Because they weaken parts of the immune system, you’re more vulnerable to infections. Tuberculosis, hepatitis B, and fungal infections can reactivate. That’s why everyone gets tested for TB and hepatitis before starting. You also need a heart check-anti-TNFs can worsen heart failure. Some people develop skin rashes, nerve problems, or even lupus-like symptoms. One Reddit user reported developing a lupus-like reaction after 18 months on Humira. Others get infusion reactions-chills, fever, or itching during treatment. These are usually mild and manageable. The biggest fear? Cancer. Studies show a small increase in lymphoma risk with long-term use, but the absolute risk remains low-about 1 in 1,000 over 10 years. The risk of untreated Crohn’s-like bowel cancer from chronic inflammation-is higher.Therapeutic Drug Monitoring: The Secret Weapon
One of the biggest advances in Crohn’s care is checking drug levels in the blood. This is called therapeutic drug monitoring. Many patients stop responding to biologics-not because the drug stopped working, but because their body cleared it too fast or made antibodies against it. Doctors now measure trough levels. For infliximab, the target is 3-7 μg/mL. For adalimumab, it’s 5-12 μg/mL. If levels are too low, the dose can be increased or given more often. If antibodies are present, switching drugs helps. Studies show patients who get monitored are 3.5 times more likely to stay in remission. This isn’t experimental. It’s standard care at top IBD centers.Real Life: What Patients Say
On Reddit’s r/Crohns_Disease community, stories are mixed but powerful. One user, CrohnWarrior87, went from 15 bowel movements a day to just 2 after three infliximab infusions. Another, IBDSurvivor22, developed a serious autoimmune reaction to Humira and spent six months recovering. A 2023 survey of over 1,200 patients found 78% felt their quality of life improved on biologics. Most could return to work. 85% stopped relying on steroids. But 65% struggled with cost. 35% had trouble scheduling infusions around their jobs. 22% found the administration process overwhelming. Support helps. IBD nurse specialists guide patients through injections, side effects, and insurance. Apps like MyIBDCoach let people track symptoms and remind them of doses. Patient assistance programs cover 30% to 50% of out-of-pocket costs for those who qualify.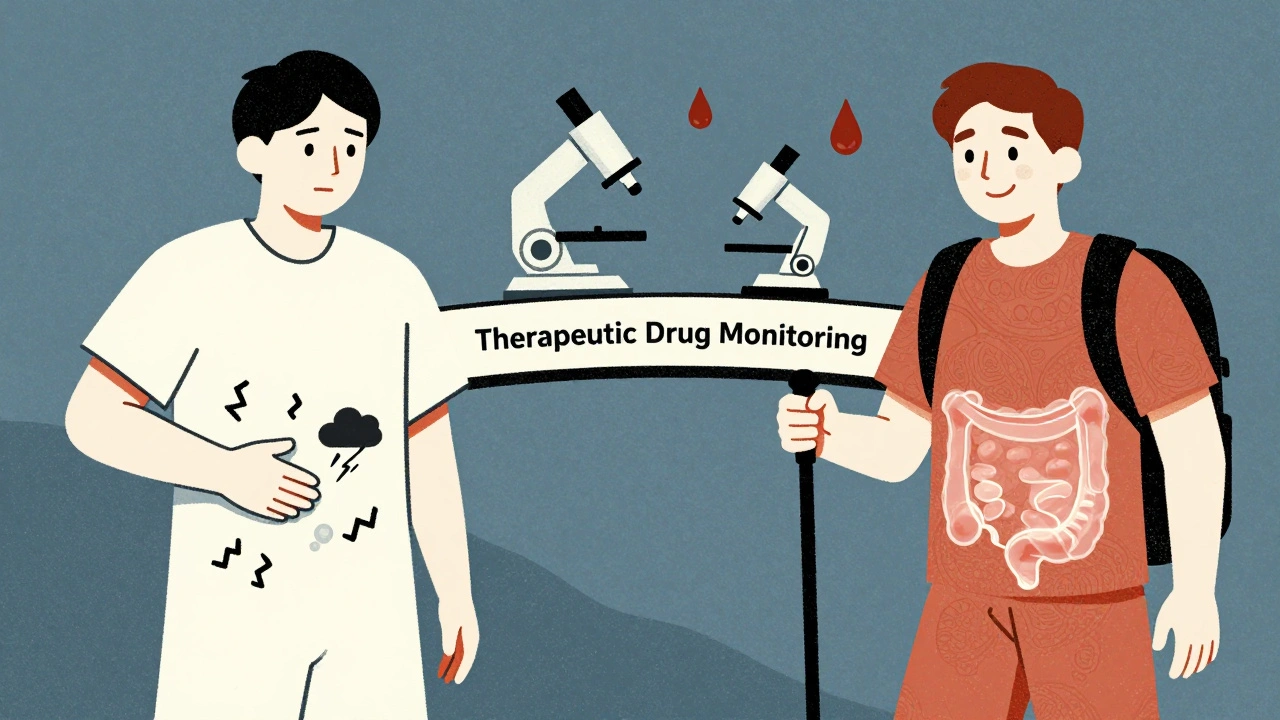
What’s Next?
New drugs are on the horizon. Ozanimod, a pill that traps immune cells in lymph nodes, showed 37% remission in trials. Mirikizumab, which blocks only IL-23 (not IL-12), had 40% endoscopic improvement. These could offer better safety profiles and oral options. The market is shifting too. Anti-TNFs still make up 65% of sales, but vedolizumab and ustekinumab are growing fast. Biosimilars will keep expanding access. The goal isn’t just to control symptoms anymore. It’s to stop the disease before it causes permanent damage.Key Takeaways
- Crohn’s disease causes chronic, deep inflammation that can lead to strictures, fistulas, and surgery if untreated.
- Biologic therapies target specific immune signals (TNF-alpha, IL-12/23, integrins) to stop inflammation at its source.
- Anti-TNF drugs (infliximab, adalimumab) are first-line for moderate-severe disease; vedolizumab and ustekinumab are alternatives based on risk profile.
- Biologics heal the gut lining, reduce hospitalizations, and cut steroid use-but cost and access remain major barriers.
- Therapeutic drug monitoring (measuring blood levels) improves outcomes and prevents treatment failure.
- Early use of biologics in high-risk patients reduces surgery risk by up to 50%.
- Side effects include infections and rare autoimmune reactions, but risks are manageable with screening and monitoring.
Can biologic therapy cure Crohn’s disease?
No, biologic therapy cannot cure Crohn’s disease. It doesn’t remove the underlying genetic or immune triggers. But it can put the disease into deep, long-lasting remission-sometimes for years. Many patients stop having symptoms, heal their gut lining, and avoid surgery. For most, biologics mean living a normal life, not just surviving flares.
How long do you need to stay on biologics?
Most people stay on biologics long-term. Stopping too soon often leads to flare-ups. Some patients who’ve been in remission for 2+ years and have healed tissue may try to taper under close supervision. But relapse rates are high-up to 60% within a year. For most, continuing treatment is the safest way to prevent damage.
Are biosimilars as good as the original biologics?
Yes. Biosimilars like Inflectra and Renflexis are nearly identical to their brand-name counterparts (infliximab). They’re tested in large studies to prove they work the same way and have the same safety profile. The FDA approves them only after rigorous review. Many insurance plans now push for biosimilars first because they cost less. Switching from brand to biosimilar is safe and common.
Do biologics cause weight gain or loss?
Biologics themselves don’t directly cause weight changes. But many patients gain weight after starting treatment because their inflammation is under control. They can eat normally again, absorb nutrients better, and regain muscle. Some lose weight if they have side effects like nausea or infections. Weight changes are usually a sign of improved disease control-not a direct effect of the drug.
Can you get vaccinated while on biologics?
Yes, but timing matters. You should get all routine vaccines (flu, pneumonia, shingles, COVID-19) before starting biologics if possible. Once on treatment, live vaccines (like MMR or yellow fever) are not safe. Inactivated vaccines are fine and strongly recommended. Always check with your IBD team before getting any shot.
What if biologics stop working?
Loss of response is common-up to 46% per year with anti-TNFs. The first step is checking drug levels and antibodies. If levels are low, increasing the dose or shortening the interval often helps. If antibodies are high, switching to a different class-like vedolizumab or ustekinumab-is the next step. Newer drugs like mirikizumab are options for those who’ve tried multiple biologics.
Is it safe to use biologics during pregnancy?
Yes, most biologics are considered safe during pregnancy. Infliximab and adalimumab cross the placenta, so doctors may stop them in the third trimester to reduce infant infection risk. Vedolizumab and ustekinumab cross less and are often continued. Keeping Crohn’s in remission during pregnancy is far safer than having a flare. Always plan ahead with your IBD team before conceiving.
Next Steps if You’re Starting Biologics
- Get screened for TB, hepatitis, and heart health before your first dose.
- Ask about therapeutic drug monitoring-don’t wait until you feel worse.
- Learn self-injection techniques with a nurse. Practice makes it easier.
- Sign up for patient assistance programs if cost is an issue.
- Use a symptom tracker app to spot flares early.
- Keep all follow-up appointments-even if you feel fine.
- Never stop or skip doses without talking to your doctor.



Deborah Andrich
December 11, 2025 AT 19:37Also, get your trough levels checked. I didn't know that was a thing until I was in remission and my doc said my levels were zero. Turns out my body was eating the drug. Dose increase fixed it.
Rawlson King
December 12, 2025 AT 02:41Alvin Montanez
December 13, 2025 AT 00:03Tommy Watson
December 14, 2025 AT 09:02Karen Mccullouch
December 16, 2025 AT 06:48Cole Newman
December 17, 2025 AT 23:16Casey Mellish
December 19, 2025 AT 16:48Tyrone Marshall
December 20, 2025 AT 03:27Emily Haworth
December 21, 2025 AT 01:37Tom Zerkoff
December 21, 2025 AT 03:47Yatendra S
December 21, 2025 AT 10:05