When a patient takes a generic pill, they expect the same effect as the brand-name version. But what if that pill isn’t made the same way? For many clinicians, that’s no longer a hypothetical question-it’s a daily concern.
What’s really inside that generic pill?
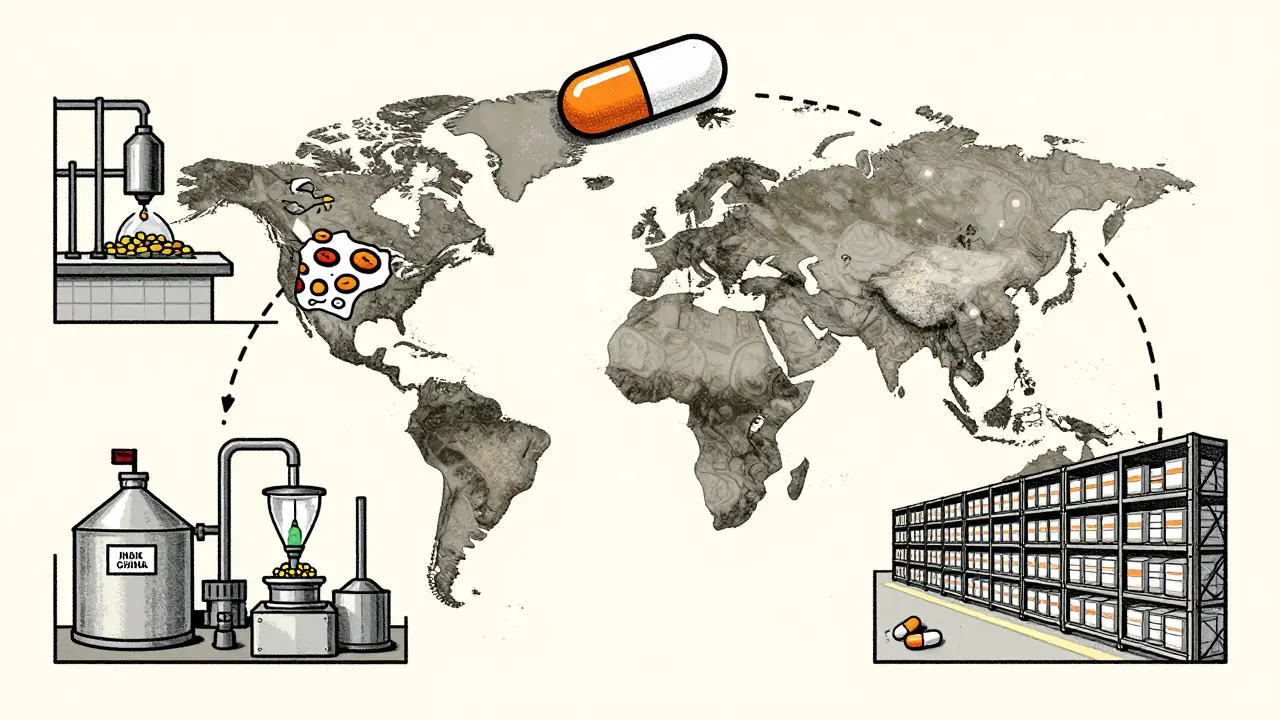
Generic drugs are supposed to be exact copies of brand-name medicines. Same active ingredient. Same dose. Same effect. That’s the promise. But the truth is, the pill you get at the pharmacy might have been assembled across three different countries. The active ingredient might come from a factory in India. The filler and coating could be added in China. The final packaging? Maybe in Germany. Only one company’s name appears on the label. The rest? Invisible. This fragmented supply chain is standard now. And it’s where quality starts to slip. A 2023 study from Ohio State University analyzed over 1.2 million adverse event reports from the FDA. The results were stark: generic drugs made in India had a 54% higher rate of severe side effects-including hospitalizations, disability, and death-compared to identical generics made in the U.S. The difference wasn’t random. It was strongest in older, low-cost generics, where price pressure has pushed manufacturers to cut corners.Why does location matter so much?
The FDA says the U.S. drug supply is among the safest in the world. And technically, they’re right. But their inspections don’t treat every factory the same. In the U.S., inspectors show up unannounced. They walk in, check records, look at equipment, and talk to workers without warning. Overseas? Inspections are scheduled months in advance. Manufacturers know exactly when they’re coming. They clean up. They fix the obvious problems. The hidden ones? They stay hidden. Professor Robert S. Gray, lead author of the Ohio State study, put it bluntly: “It’s like doing a home inspection while the homeowner is home cleaning for you.” That’s not just a metaphor-it’s a systemic flaw. A 2023 report from the Duke-Margolis Center found that outdated equipment, poor quality control, and lack of real-time monitoring are common in overseas facilities making low-cost generics. These aren’t new problems. They’re the result of a 40-year shift toward globalized, low-cost manufacturing.Older generics, bigger risks
Not all generics are created equal. The older the drug, the higher the risk. Why? Because when a drug’s patent expires, dozens of companies jump in to make it. Prices drop fast. Profit margins vanish. The only way to stay profitable? Cut costs. That means using cheaper raw materials, reducing testing, skipping maintenance on aging machinery. One drug, 20 manufacturers, one race to the bottom. Drugs like levothyroxine, metformin, and warfarin-medications patients rely on daily-have seen the most complaints. These aren’t flashy new treatments. They’re the backbone of chronic disease management. A tiny variation in absorption can mean a patient’s thyroid levels swing dangerously, their blood sugar spikes, or their clotting time goes off the charts. Clinicians see it: patients who’ve been stable for years suddenly have unexplained symptoms after a switch to a new generic batch. No one knows why. No one tracks it well.
The FDA’s role-and its limits
The FDA has over 1,300 staff dedicated to drug quality. They inspect hundreds of facilities every year. They require generics to prove bioequivalence-meaning the drug enters the bloodstream at the same rate and amount as the brand. But bioequivalence doesn’t guarantee safety. It doesn’t catch impurities. It doesn’t monitor long-term stability. It doesn’t track what happens when a tablet sits on a warehouse shelf in a humid country before being shipped to the U.S. The agency’s own data shows that 80% of drug shortages between 2015 and 2023 were tied to manufacturing problems. And most of those shortages involved older generics. The Duke-Margolis Center calls this “the leading cause of treatment delays”-especially for cancer patients, transplant recipients, and people on life-sustaining medications. When a batch fails inspection overseas, production halts. No backup. No local supply. Patients go without.Can technology fix this?
There’s a solution hiding in plain sight: advanced manufacturing technologies (AMTs). Things like continuous manufacturing-where the drug is made in one sealed, automated line instead of scattered steps-and real-time quality monitoring, where sensors check purity and potency as the drug is made. These systems reduce errors, cut waste, and make it nearly impossible to hide quality issues. Here’s the catch: 80% of AMT-made drugs are produced in the U.S. Why? Because the upfront cost is high. A single continuous manufacturing line can cost $50 million. For a company making pennies per pill, that’s not a smart investment. So they stick with old, cheap, manual processes overseas. The result? A two-tiered system: high-quality generics made domestically for niche markets, and low-cost, high-risk generics flooding the rest of the system.
What clinicians are seeing-and saying
Clinicians aren’t just worried. They’re documenting it. Pharmacists in hospitals report patients reacting differently to the same generic drug from different batches. One patient’s blood pressure stabilizes with Generic A from a U.S. supplier. Switch them to Generic B from India? Their numbers spike. They’re told it’s the same drug. But the patient doesn’t care about the label. They care about feeling sick. Dr. Iyer, a prescribing clinician, says: “We need to incentivize quality, not just price.” He’s started asking his pharmacy: “Where was this made?” If they don’t know, he refuses to prescribe it. Other doctors are doing the same. It’s not about being anti-generic. It’s about being pro-safety. The NIH published a blunt conclusion: “Is the quality of generic drugs cause for concern?” Answer: yes. From a pharmacist’s perspective. That’s not an outlier opinion. It’s a growing chorus.Domestic production: a realistic fix?
The University of Wisconsin School of Pharmacy says it plainly: “If we have more generic manufacturing happening domestically, we would ideally have fewer quality concerns, fewer shortages, and a more stable supply chain.” It sounds simple. And it is. But it’s not cheap. Rebuilding U.S. generic manufacturing would require investment-government incentives, tax breaks, guaranteed purchase agreements. Right now, the market punishes quality. The lowest bidder wins. That needs to change. One proposal? Make the country of manufacture visible on the label. Let prescribers and patients choose. Let the market reward transparency. The FDA has started pilot programs for advanced manufacturing. But without pressure from clinicians, patients, and lawmakers, progress is slow. And every day that passes, more patients are getting pills made under conditions we can’t fully see-or trust.What you can do
If you’re a clinician: ask where your generics come from. Document reactions. Report adverse events-even if you think it’s “just a generic.” The FDA’s reporting system relies on you. If you’re a patient: if you notice a change in how a generic medication affects you after a refill, speak up. Ask your pharmacist: “Is this the same brand as last time?” Keep a log. Your experience matters. This isn’t about rejecting generics. It’s about demanding better. Cheap shouldn’t mean risky. Accessible shouldn’t mean unsafe. The system was built to save money. But if it’s costing lives, it’s time to rebuild it.Are generic drugs always safe?
Generic drugs are required to meet FDA standards for bioequivalence, meaning they deliver the same active ingredient as brand-name drugs. But bioequivalence doesn’t guarantee identical safety or long-term stability. Studies show higher rates of severe adverse events with generics made overseas, especially older drugs produced under low-cost conditions. Quality varies by manufacturer, location, and batch.
Why are generics made overseas cheaper?
Manufacturing costs are lower in countries like India and China due to lower labor wages, fewer regulatory enforcement costs, and less investment in modern equipment. Companies compete on price, so they cut corners on maintenance, testing, and quality control. This drives down the cost of the final product-but increases risk.
Does the FDA inspect overseas factories?
Yes, but inspections are scheduled in advance, unlike unannounced inspections in the U.S. This gives manufacturers time to prepare, clean up, and hide ongoing issues. Experts argue this undermines the ability to detect real problems. Only about 14% of active pharmaceutical ingredients are made in the U.S., meaning most inspections happen under conditions that favor concealment.
What’s the difference between bioequivalence and quality?
Bioequivalence means the drug enters your bloodstream at the same rate and amount as the brand-name version. Quality refers to purity, stability, consistency, and freedom from harmful impurities. A drug can be bioequivalent but still contain contaminants, degrade faster, or vary between batches. Bioequivalence is a minimum standard-not a guarantee of safety.
Can advanced manufacturing improve generic drug quality?
Yes. Technologies like continuous manufacturing and real-time monitoring reduce human error, improve consistency, and catch problems as they happen. Over 80% of drugs made with these technologies are produced in the U.S., where oversight is stronger. The challenge is cost-these systems require millions in upfront investment, which most low-cost generic manufacturers avoid.
Should I avoid generic drugs altogether?
No. Generic drugs save billions in healthcare costs and are safe for most people. But if you notice new side effects after switching brands, or if you’re on a critical medication like blood thinners or thyroid hormone, ask your pharmacist where it’s made. Choose generics from U.S. manufacturers when possible. Don’t assume all generics are equal.



Lynsey Tyson
December 19, 2025 AT 09:24It’s wild how we just accept that our meds come from who-knows-where. I’ve been on metformin for years and never thought to ask where it was made. Now I’m kinda freaked out.
Dikshita Mehta
December 20, 2025 AT 10:56As someone working in pharma quality control in India, I can say this isn’t about nationality-it’s about the company. We have world-class facilities here that outperform some U.S. plants. The problem is the race to the bottom. Not all Indian manufacturers cut corners. But the ones that do? They’re the ones selling to the lowest bidder.
Dominic Suyo
December 21, 2025 AT 23:51This whole thing is a corporate circus. The FDA’s a rubber stamp for Big Pharma’s offshore outsourcing. They inspect factories like they’re checking a PowerPoint deck-nice slides, zero substance. Meanwhile, patients are guinea pigs in a global cost-cutting experiment.
William Storrs
December 22, 2025 AT 12:50Don’t panic. Most generics are fine. But if you’re on warfarin or thyroid meds? Pay attention. Track your labs. If you switch brands and feel off, speak up. Your voice is the only thing that pushes change.
Dorine Anthony
December 22, 2025 AT 21:15I’ve seen this in my hospital. One batch of levothyroxine makes patients jittery. Another makes them exhausted. We don’t track the manufacturer on the chart. We should. It’s not conspiracy-it’s clinical.
Andrew Kelly
December 23, 2025 AT 19:45Oh please. You think the U.S. is some pristine pharma paradise? The FDA approves 90% of applications without even reading the data. And let’s not forget the 2018 tainted valsartan recall-made in China, but approved by the FDA. This whole narrative is just fearmongering dressed up as concern.
Anna Sedervay
December 24, 2025 AT 11:47One must contemplate, with grave solemnity, the ontological implications of pharmaceutical sovereignty. When the pill one ingests is a composite of geopolitical indifference, rendered invisible by corporate obfuscation, we are no longer patients-we are data points in a neoliberal dystopia. The FDA, in its bureaucratic inertia, has abdicated its moral duty. The label must bear the factory’s coordinates. Not merely the brand. The *exact* coordinates.
Gloria Parraz
December 25, 2025 AT 06:03I can’t believe we’re still having this conversation. We know the system is broken. We know people are getting sick. And yet nothing changes. This isn’t about money. It’s about who we value. Are we willing to pay a little more to keep someone alive? Or do we just want the cheapest thing on the shelf and pretend it’s safe?
Nicole Rutherford
December 26, 2025 AT 19:02Wow. So now we’re blaming India? Funny how the same people who scream about outsourcing when it’s jobs are suddenly fine with it when it’s their meds. Wake up. The problem isn’t the country-it’s the greed. And you’re all just enabling it by buying the cheapest option.
James Stearns
December 28, 2025 AT 00:16It is an incontrovertible fact that the modern pharmaceutical supply chain is a grotesque caricature of capitalist efficiency. The notion that a tablet can be assembled across three continents and still be deemed ‘bioequivalent’ is not only scientifically dubious-it is ethically indefensible. The FDA’s standards are relics. We require a new paradigm. One based on transparency. Accountability. And, dare I say, dignity.
Nina Stacey
December 28, 2025 AT 13:09My grandma switched to a new generic and started getting dizzy. We didn’t think much of it till she had a fall. Turned out the new batch had a different filler and her kidneys couldn’t handle it. We switched back to the old one and she’s fine. No one told us the batch changed. No one even asked. If you’re on meds, don’t just take them-know them. Write it down. Ask questions. Your life might depend on it
Ashley Bliss
December 30, 2025 AT 10:48This isn’t just about pills. It’s about trust. We’ve been sold a lie that medicine is science, pure and objective. But it’s not. It’s business. And when your life depends on a pill made in a factory where the workers don’t even know what they’re producing, you’re not being treated-you’re being exploited. The system doesn’t care if you live or die. It only cares if you keep buying. And we keep buying. Because we’re told it’s safe. Because we’re told it’s the same. But it’s not. And we’re all just pretending.
Mark Able
December 30, 2025 AT 18:52My pharmacy just started labeling the country of origin. I didn’t even know they could. Now I’m choosing the U.S.-made ones even if they’re $3 more. I’d rather pay extra than risk my health. If enough of us do this, companies will have to change. It’s that simple.