Pancreatic Cancer Treatment Combination Calculator
This tool helps estimate which immunotherapy combinations might be most effective based on your biomarker profile. Note that this is for informational purposes only and should not replace professional medical advice.
When you hear the phrase pancreatic cancer, the first thing that often comes to mind is a grim prognosis. Yet, behind those stark statistics lies a sophisticated battle between rogue cells and the body’s own defense forces. Understanding how the immune system meets pancreatic cancer-and why it usually loses-opens the door to new treatment ideas that could shift those odds.
Why pancreatic cancer is a master of disguise
Pancreatic cancer is a type of malignant tumor that originates in the pancreas, an organ that produces digestive enzymes and hormones like insulin. What makes it especially tough is its ability to hide from immune surveillance. The tumor quickly builds a protective shield called the tumor microenvironment (TME), a dense network of fibroblasts, blood vessels, and immune cells that collectively suppress attacks.
Scientists have identified a handful of tricks the cancer uses: mutating key genes such as KRAS, secreting immunosuppressive cytokines like TGF‑β, and recruiting cells that act as bodyguards for the tumor.
Key immune players that get sidelined
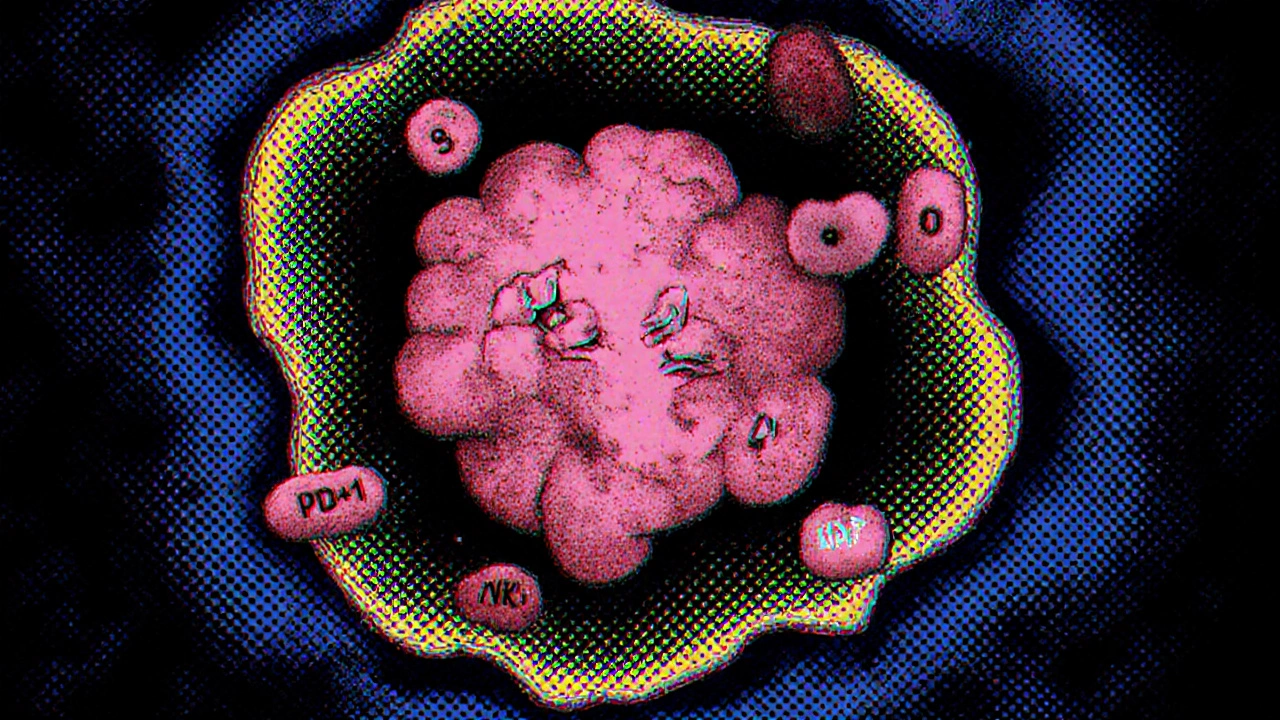
The immune system is a network of cells, tissues, and organs that protect us from disease. In a healthy setting, T cells-especially cytotoxic CD8⁺ cells-recognize abnormal proteins on cancer cells and launch a kill‑mission. Natural killer (NK) cells and macrophages also patrol the pancreas, ready to engulf suspicious cells.
In pancreatic cancer, however, these defenders are either exhausted or reprogrammed. The TME releases high levels of interleukin‑10 (IL‑10) and prostaglandin E2, which push T cells into an “exhausted” state marked by PD‑1 and CTLA‑4 receptors. When these checkpoints are engaged, the T cells essentially turn off, letting the tumor grow unchecked.
Cellular roadblocks in the tumor microenvironment
Three cell types dominate the immunosuppressive landscape:
- Cancer‑associated fibroblasts (CAFs) lay down a thick collagen matrix that physically blocks immune cell infiltration.
- Myeloid‑derived suppressor cells (MDSCs) emit arginase and reactive oxygen species that dampen T‑cell activity.
- Regulatory T cells (Tregs) produce cytokines that further silence cytotoxic responses.
All these actors together create a hostile neighborhood where even the most vigilant immune cells struggle to survive.
How immunotherapy is trying to tip the balance
Over the past decade, researchers have tried to break through the TME’s defenses with several immunotherapy strategies. Below is a quick glance at the main approaches being tested for pancreatic cancer.
| Approach | Mechanism | Clinical status (2025) | Key pros | Key cons |
|---|---|---|---|---|
| Checkpoint inhibitors | Block PD‑1/PD‑L1 or CTLA‑4 to reactivate exhausted T cells | Phase II trials; modest single‑agent activity | Well‑understood; oral or IV dosing | Low response rate when used alone |
| CAR‑T therapy | Engineer patient T cells to target tumor‑specific antigens (e.g., mesothelin) | Early Phase I/II; promising in selected patients | Potential for durable remission | Manufacturing complexity; cytokine release risk |
| Vaccines (e.g., KRAS‑mutant peptide) | Prime the immune system to recognize mutant proteins | Phase II ongoing; mixed results | Low toxicity; adaptable to patient’s mutation | Often requires booster doses |
| Oncolytic viruses | Virus infects tumor cells, releases antigens, and stimulates immunity | Phase I/II; early safety data positive | Can remodel TME physically | Delivery challenges; immune neutralization |
While each approach has its own hurdles, the trend is clear: single‑agent immunotherapy rarely works in pancreatic cancer. Instead, scientists are stacking therapies-combining checkpoint blockers with chemotherapy, or pairing CAR‑T cells with drugs that deplete MDSCs-to create a multi‑front assault.
Combination strategies that show promise
One hot area in 2025 is the pairing of checkpoint inhibitors with agents that remodel the TME. For example, hyaluronidase enzymes can break down the dense stroma, allowing T cells to penetrate deeper. Another avenue involves using radiation to increase tumor antigen presentation, effectively turning a “cold” tumor hot.
Early data from the POLO‑Combo trial indicate that adding a PD‑1 blocker to the standard gemcitabine‑nab‑paclitaxel regimen improves median progression‑free survival by about three months. Though not a cure, it suggests that the right cocktail can extend quality life.

Looking ahead: biomarkers and personalized immunotherapy
Scientists agree that the future hinges on better patient selection. Biomarkers such as high tumor mutational burden (TMB), presence of neoantigens from KRAS mutations, and the density of infiltrating CD8⁺ T cells are being studied as predictors of response.
Liquid biopsies that measure circulating tumor DNA (ctDNA) and immune cell signatures could soon let clinicians adjust therapy in real time, moving away from the one‑size‑fits‑all model.
Practical takeaways for patients and caregivers
- Ask your oncologist whether you’re eligible for clinical trials that test combination immunotherapy.
- Maintain a healthy diet and moderate exercise; these can modestly improve immune function.
- Stay informed about biomarkers; a simple blood test might open doors to targeted immunotherapy.
- Connect with support groups-sharing experiences helps you navigate complex treatment decisions.
While the battle against pancreatic cancer is still fierce, the growing understanding of how the immune system interacts with the disease means new weapons are being added to the arsenal every year.
Frequently Asked Questions
Can checkpoint inhibitors cure pancreatic cancer?
As of 2025, checkpoint inhibitors alone rarely lead to long‑term remission in pancreatic cancer. They work best when combined with chemotherapy, radiation, or agents that break down the tumor’s stromal barrier.
What is the role of KRAS mutations in immunotherapy?
KRAS is mutated in over 90% of pancreatic tumors. These mutations create unique neoantigens, which can be targeted by personalized vaccines or engineered T‑cell receptors, potentially improving immune recognition.
Are CAR‑T cells safe for pancreatic cancer patients?
Early‑phase trials show CAR‑T therapy can be administered safely, but it carries risks like cytokine release syndrome. Ongoing studies aim to fine‑tune dosing and target antigens such as mesothelin to reduce side effects.
How do lifestyle choices affect the immune response to pancreatic cancer?
Regular moderate exercise, a diet rich in vegetables, and adequate sleep can modestly boost immune surveillance. While they won’t replace medical treatment, they support overall resilience.
What clinical trials are currently recruiting for pancreatic cancer immunotherapy?
The NCI lists dozens of active trials, including those combining PD‑1 inhibitors with hyaluronidase, KRAS‑directed vaccines, and oncolytic virus platforms. Checking clinicaltrials.gov with your location will give the most up‑to‑date list.





barnabas jacob
October 20, 2025 AT 16:20The tumor microenvironment (TME) in pancreatic adenocarcinoma manifests an immunosuppressive cytokine milieu that effectively neutralizes checkpoint blockade efficacy. It's astonishing how many clinicians still tout monotherapy as a panacea despite the well‑documented KRAS‑driven immune evasion pathways. One could argue that ignoring the dense CAF scaffold is tantamount to willful negligence in a field bristling with translational data. Ultimately, the moral imperative is to champion combination regimens or risk perpetuating therapeutic nihilism.
jessie cole
October 20, 2025 AT 20:30Dear readers, let us take a moment to appreciate the tremendous progress made in understanding pancreatic cancer’s interaction with the immune system. Your curiosity is the catalyst that fuels scientific breakthroughs, and every insight you gain brings us one step closer to hope. Remember, perseverance in the face of daunting odds is the hallmark of true bravery. Together, we can transform despair into determination, and knowledge into life‑saving action.
Kirsten Youtsey
October 21, 2025 AT 00:06It is abundantly clear to the discerning observer that the mainstream narrative surrounding immunotherapy is meticulously curated by a consortium of vested interests. The selective publication of marginally positive phase II data serves as a smokescreen, diverting attention from the underlying molecular inertia inherent in pancreatic neoplasms. One must question whether the reported modest improvements are merely statistical artifacts rather than genuine therapeutic breakthroughs. Moreover, the emphasis on checkpoint inhibitors eclipses the far more nuanced, albeit less marketable, strategies involving stromal modulation. In essence, the current discourse reflects an intellectual complacency that belies the complexity of the disease.
Matthew Hall
October 21, 2025 AT 01:13The whole thing reeks of a clandestine lab rat experiment gone rogue.
Vijaypal Yadav
October 21, 2025 AT 04:50From a mechanistic standpoint, the presence of high tumor mutational burden (TMB) correlates with increased neoantigen presentation, which in turn can potentiate T‑cell recognition when combined with PD‑1 blockade. Additionally, quantifying circulating tumor DNA (ctDNA) offers a minimally invasive metric to monitor clonal evolution and therapeutic response in real time. Studies have demonstrated that hyaluronidase-mediated stromal degradation enhances intratumoral infiltration of CD8⁺ lymphocytes, thereby synergizing with checkpoint inhibition. It is also worth noting that mesothelin‑targeted CAR‑T cells, while still in early‑phase trials, have exhibited a favorable safety profile compared with traditional systemic chemotherapy. Collectively, these data underscore the necessity of a multi‑modal approach tailored to individual biomarker signatures.
Ron Lanham
October 21, 2025 AT 09:00It is a moral failing of our healthcare system to continue to present pancreatic cancer as an untreatable death sentence when the scientific literature offers a compendium of actionable strategies. The immune landscape, though formidable, is not an impenetrable fortress; rather, it is a dynamic arena where judiciously combined therapies can tip the scales. First, the dense desmoplastic stroma, rich in collagen and hyaluronan, must be enzymatically remodeled to allow effector cells to traverse the tumor microenvironment. Second, checkpoint inhibition should be administered in concert with agents that deplete myeloid‑derived suppressor cells, thereby relieving T‑cell exhaustion. Third, personalized vaccine platforms targeting KRAS neoantigens provide the necessary priming signal to direct cytotoxic lymphocytes toward malignant cells. Fourth, the incorporation of low‑dose radiation serves a dual purpose of augmenting antigen release and upregulating MHC class I expression on tumor cells. Fifth, emerging data on oncolytic viruses reveal that viral lysis not only reduces tumor burden but also acts as an in situ vaccine, further galvanizing the immune response. Moreover, the ethical imperative extends beyond molecular tinkering to encompass equitable access to clinical trials for underserved populations. The disregard for socioeconomic barriers exacerbates disparities and contravenes the principle of justice that underlies medical practice. It is incumbent upon oncologists to communicate transparently about the realistic benefits and risks of each investigational modality. Patients, too, bear a responsibility to engage actively with their care teams and to seek out reputable sources of information. In doing so, they empower themselves against the fatalistic narratives that have long plagued pancreatic cancer discourse. Likewise, funding agencies must prioritize translational research that bridges bench discoveries with bedside applications. The stagnation of monotherapy trials is a testament to the futility of singular approaches in the face of a multifaceted disease. Comprehensive, adaptive trial designs that allow for real‑time biomarker‑driven modifications epitomize the progressive mindset necessary for breakthroughs. Ultimately, the convergence of scientific rigor, moral responsibility, and collaborative spirit will forge a path toward meaningful survival gains for patients confronting this relentless malignancy.
Andrew Hernandez
October 21, 2025 AT 13:10Thank you for the thorough overview It highlights how precision medicine can be culturally sensitive while respecting patient autonomy We appreciate the balanced view without excessive technical jargon
Sebastian Green
October 21, 2025 AT 17:20I hear the frustration many patients feel when treatments seem to stall, and it’s understandable to feel overwhelmed by the complexity. Knowing that the immune system can be coaxed into action offers a glimmer of hope, even if progress feels incremental. Your dedication to staying informed and supporting loved ones makes a real difference in navigating this journey. Keep leaning on community resources; shared experiences often provide the comfort that data alone cannot.
Wesley Humble
October 21, 2025 AT 22:53While the emotional resonance is commendable, the empirical evidence still indicates that combination immunotherapy yields only marginal improvements in median progression‑free survival 😑. The cited POLO‑Combo trial, for instance, fails to demonstrate a statistically significant overall survival benefit, underscoring the necessity for rigorous endpoint analysis 📊. Moreover, the reliance on surrogate biomarkers such as TMB without robust validation may lead to over‑optimistic patient expectations. It is imperative that stakeholders refrain from hyperbolic rhetoric and adhere to data‑driven conclusions 🧐.